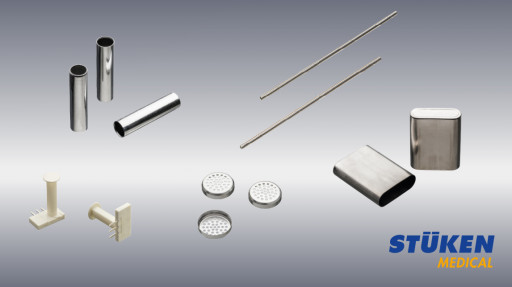
ST\u00dcKEN Components for the Medical Industry
ST\u00dcKEN supplies its customers with reliable components for medical tools, for the application of medicines, for primary packaging and for medical housings.
FOUNTAIN INN, S.C. - March 7, 2022 - (Newswire.com)
Miniaturization and standardization have been driving the rising demand for deep-drawn metal components in the medical industry. STÜKEN North America has successfully expanded its business in this area. Packaging solutions for medical devices and drug delivery have become a significant mainstay. The company aims to strengthen its position further by achieving certification according to ISO 13485. First steps towards implementation have been taken.
The US healthcare market is growing, and it is growing fast. According to the Centers for Medicare & Medicaid Services, national health spending is projected to reach $6.2 trillion by 2028. One factor driving this growth is the aging population. Older people are more susceptible to catching diseases. Another driver is the trend towards self-medication and OTC ("over-the-counter") products. As a consequence, products are designed for easy handling, for example by making components smaller. With rising demand, mass production becomes economical - and so does deep drawing. In recent years, STÜKEN North America has successfully expanded its business with packaging solutions for medical devices and drug delivery. Last year, the South Carolina-based company received the "Best Supplier Award 2021" from one of their medical customers.
"The share of sales with medical parts will continue to increase in the coming years. In order to be even better attuned to the needs and requirements of this market, we recently started preparing for the ISO 13485 certification," explains Dr. Dennis Gossmann, Vice President and General Manager of STÜKEN North America. "With this certification, we create additional confidence and make it easier for our customers to get approval for their medical devices. With quality assurance, conformity to standards, complete documentation and traceability we prove that we are a reliable partner."
Further investments in technologies for the medical market are planned for the coming years. This includes cleaning and finishing processes. That way, STÜKEN North America can offer everything from a single source: Ready-to-use solutions from an efficient and largely internal process chain with extremely high and reliable quality. On top of this, a dedicated team makes sure that the focus is always on the customer's requirements, for example, with comprehensive customer service and innovative technical support in the development phase. "The certification according to ISO 13485 and the investment into new technology are part of the STÜKEN group´s global commitment to the medical industry. As part of this network, we can draw on the extensive know-how from our already certified German headquarters," says Gossmann.
Press contact
Hubert Stüken GmbH & Co. KG
Sandra Göhner-Baake, Marketing Manager, Alte Todenmanner Straße 42, 31737 Rinteln, Germany
Phone: +49 5751 702 0, marketing@stueken.de
Press Release Service by Newswire.com
Original Source: Deep Drawing Specialist STÜKEN North America Announces First Steps Towards ISO 13485 Certification













