A team led by the U.S. Department of Energy’s (DOE) Argonne National Laboratory has developed a new catalyst composed of elements abundant in the Earth. It could make possible the low-cost and energy-efficient production of hydrogen for use in transportation and industrial applications. Clean hydrogen could not only propel vehicles with no emitted pollutants but also help decarbonize industrial processes.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230530005654/en/
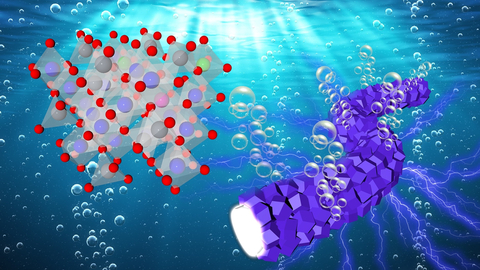
Oxygen bubbles evolving from fibrous, interconnected catalyst particles (right) during electrocatalytic reaction with water. Lattice structure for cobalt-based catalyst on left. (Image by Argonne National Laboratory/Lina Chong and Longsheng Wu using a Shutterstock background.)
Other contributors to the project include DOE’s Sandia National Laboratories and Lawrence Berkeley National Laboratory, as well as Giner Inc.
Proton exchange membrane (PEM) electrolyzers represent a new generation of technology for hydrogen production. They can split water into hydrogen and oxygen with high efficiency at near room temperature. The reduced energy demand makes them an ideal choice for producing clean hydrogen by using renewable but intermittent sources, such as solar and wind.
This electrolyzer runs with separate catalysts for each of its electrodes (cathode and anode). The cathode catalyst yields hydrogen, while the anode catalyst forms oxygen. A problem is that the anode catalyst uses iridium, which has a current market price of around $5,000 per ounce. The lack of supply and high cost of iridium pose a major barrier for widespread adoption of PEM electrolyzers. The main ingredient in the team’s new catalyst is cobalt, which is substantially cheaper than iridium.
Giner Inc., a leading research and development company working toward commercialization of electrolyzers and fuel cells, evaluated the new catalyst using its PEM electrolyzer test stations under industrial operating conditions. The performance and durability far exceeded that of competitors’ catalysts.
The team’s achievement is a step forward in DOE’s Hydrogen Energy Earthshot initiative, which mimics the U.S. space program’s “Moon Shot” of the 1960s. Its ambitious goal is to lower the cost for green hydrogen production to one dollar per kilogram in a decade. Production of green hydrogen at that cost could reshape the nation’s economy. Applications include the electric grid, manufacturing, transportation and residential and commercial heating.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230530005654/en/
Contacts
Christopher J. Kramer
Head of Media Relations
Argonne National Laboratory
media@anl.gov
Office: 630.252.5580













